Background
Recent update of the VIALE-A study showed a long-term survival for approximately 30% of acute myeloid leukemia (AML) patients treated with venetoclax and azacitidine (VEN-AZA) for a newly-diagnosed AML (ND-AML) (Pratz K.W et al., ASH 2022). A recent report of a small cohort of responding patients who interrupted VEN-AZA displayed a sustained treatment-free remission (TFR) period suggesting that treatment interruptions might be considered (Chua CC. et al., Blood Adv. 2022). To evaluate the impact of VEN-AZA discontinuation on survival and time without treatment, we retrospectively analyzed the characteristics and outcomes of patients who stopped VEN and/or AZA for reasons other than progression (e.g. hematological or other toxicities). We presented the first 51 patients from our cohort last year at ASH meeting (Garciaz S. et al, ASH 2022). In this abstract, we analyzed separately ND-AML and relapsed/refractory (R/R) AML.
Methods
Eligible patients 1) have been treated with VEN-AZA for a ND or R/R AML, 2) have stopped AZA and/or VEN for more than 3 months, 3) are in remission (CR, CRi or Morphologic leukemia-free state, [MLFS]) at the time of treatment interruption, 4) are ≥ 18-year-old. Main endpoints were overall survival (OS) and treatment-free remission (TFR) calculated from the time between the last day of VEN to relapse, retreatment, or death. We measured the impact of prognostic factors on OS by a Cox regression taking disease status (ND vs R/R), the discontinued treatment (VEN vs VEN-AZA), the cytogenetics and NPM1 or IDH mutations.
Results
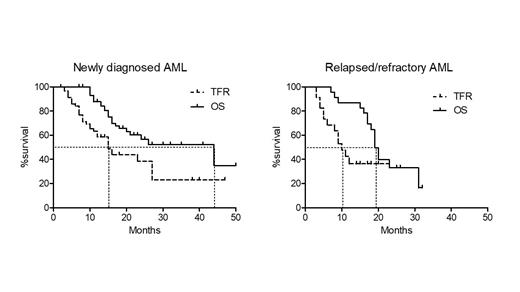
We included 83 patients from French Innovative Leukemia Organization (FILO) centers and Moffit Cancer Center (US) treated with VEN-AZA for a ND-AML or a R/R AML between 11/18 and 07/23. Regarding the ND-AML patients (n=60; 32 males and 28 females), the median age was 76 (range, 26-86.5), median platelet count, WBC, ANC and bone marrow blast involvement was 52 G/L (range, 9-296), 2.8 G/L (range, 0.6-200), 0.6 G/L (range, 0.02-31.6) and 40% (range, 10-99), respectively. Cytogenetics was intermediate (int.) in 48 cases (80%) and unfavorable (unfav.) in 12 patients (20%). Eleven AML (18.3%) had NPM1 mutations, and 19 (31.6%), IDH alterations including 7 IDH1, 8 IDH2R140 and 4 IDH2R172. Six (10%) and 5 (8.3%) patients had FLT3 or TP53 mutations, respectively. The median number of VEN-AZA cycle was 4 (1-17). The best response was CR (n=44, 73.3%), CRi (n=13, 18.3%) and MLFS (n=3, 5%). Twenty-one patients out of 25 (78%) reached MRD negativity assessed by molecular (n=11) or flow cytometry (n=10) techniques. Twenty-six patients (43.3%) stopped VEN-AZA and 34 (56.7%) stopped VEN and continued AZA monotherapy for a median number of 7 AZA cycles (1-41). Reasons for treatment discontinuation were prolonged cytopenia, non-hematological toxicities, patient choice or unknown in 35 (58%), 6 (10%), 8 (13.3%), and 11 (18.3%) patients, respectively. We registered 27 relapses and 24 deaths. Fourteen patients were retreated including 11 patients who were rechallenged with VEN-AZA. Second CR/CRi rate with VEN-AZA was 3/11 (27.7%). With a median follow of 23 months, median TFR was 15 months (ranges, 3-47) and median OS was 44 months (ranges, 7-50).
We also analyzed a smaller cohort of 23 R/R responding patients who stopped AZA and/or VEN. The median age was 73, median number of previous lines was 1 (1-3). Twenty-one patients (91.3%) received prior intensive chemotherapy and 4 (17.4%) prior allogeneic hematopoietic transplantation. Cytogenetic risk was int. in 17 (73.9%) and unfav. in 5 patients (21.7%) and missing in one patient. NPM1 and IDH genes were mutated in 6 (26%) and 10 (43.4%) cases, respectively. Sixteen patients (69.6%) stopped VEN-AZA and 7 (30.4%) interrupted VEN only. Nine patients resumed a treatment including 6 patients rechallenged with VEN-AZA. Among them, 3/6 (50%) obtained a second CR/CRi. Median TFR and OS were 10 (ranges, 3-23) and 19 (ranges, 7-32), respectively.
Factor impacting OS in multivariate analysis were R/R AML disease status (Hazard Ratio [HR] =0.47, ranges, 0.23-0.87), and IDH mutations (HR= 2.1, ranges, 1.05-4.34), but not unfav. cytogenetics, NPM1 mutations, or the discontinued treatment (VEN vs VEN-AZA).
Conclusion:
Discontinuation of VEN and/or AZA in responding patients was associated with sustained responses and long-term survival rates. Resuming treatment may induce a second remission in some patients.
Disclosures
Bertoli:Abbvie: Honoraria, Other: Travel; Novartis: Honoraria; Astellas: Honoraria; BMS-Celgene: Honoraria; Servier: Honoraria; Jazz Pharmaceuticals: Honoraria, Other: Travel. Sallman:AbbVie, Affimed Gmbh, Gilead, Incyte, Intellisphere, LLC, Molecular Partners AG, PGEN Therapeutics, Inc., Takeda, Zentalis; Advisory board for AvenCell, BlueBird Bio, BMS, Intellia, Jasper Therapeutics, Kite, Magenta Therapeutics, NKARTA, Novartis, Orbita: Consultancy; Aprea, Jazz: Research Funding. Dumas:Abbvie: Honoraria; Astellas: Honoraria, Other: Research support for institution; BMS: Honoraria, Other: Research support for institution; Servier: Honoraria, Other: Research support for institution; Novartis: Honoraria, Other: Research support for institution; Daiichi-Sankyo: Honoraria, Other: Research support for institution; Jazz pharmaceutical: Honoraria; Janssen: Honoraria; Roche: Other: Research support for institution. Pigneux:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings; Gilead: Honoraria; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees. Récher:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Iqvia: Research Funding; Boehringer: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal